Abstract
BACKGROUND: Cancer associated thrombosis (CAT) guidelines recommend direct oral anticoagulants as alternatives to low molecular weight heparin (LMWHs) in most patients. We sought to compare the effectiveness and safety of rivaroxaban versus LMWH in a broad CAT cohort.
METHODS: This retrospective cohort analysis used US Optum De-Identified electronic health data from January 1, 2012 through December 31, 2020 to identify patients with any active cancer type, who were admitted to the hospital, emergency department or observation unit for venous thromboembolism (VTE) and treated with rivaroxaban or LMWH. We used propensity score overlap weighting to balance anticoagulant cohorts on baseline covariates. Hazard ratios (HRs) with 95% confidence intervals (CIs) for VTE, bleeding related hospitalization and all-cause mortality were calculated using Cox regression.
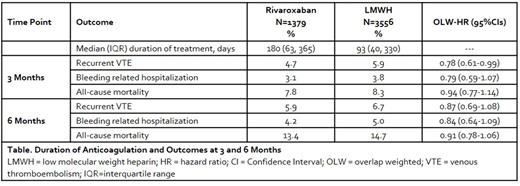
RESULTS: We identified 4935 patients treated with rivaroxaban or LMWH (Table). Of these patients, 26.5% were ≥75 years of age, 55.9% were female, 19.0% had a body mass index ≥35 kg/m2 and 18.2% had a GFR <60 mL/minute at baseline. The CAT event was a pulmonary embolism ± deep vein thrombosis in 52.0% of patients, 46.3% had metastatic disease and 60% received active cancer treatment within 4 weeks of the CAT event. Cancer types included gastrointestinal (29.4%), genitourinary (26.2%), lung (24.0%), breast (19.7%) and hematologic (14.4%). At 3 months, rivaroxaban was associated with a significant 22% relative hazard reduction in recurrent VTE versus LMWH among all cancer patients. No significant difference in bleeding related hospitalization or all-cause mortality were observed at 3 months. Directionally similar results to those at 3 months were observed at 6 months for all outcomes.
CONCLUSIONS: We observed less recurrent VTE and no increase in bleeding related hospitalizations associated with rivaroxaban compared to LMWH at 3 months in a broad cohort of patients with various cancer types.
Disclosures
Coleman:Medscape: Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding; Bayer AG: Consultancy, Honoraria, Other: Support for attending meetings and/or travel, Research Funding; Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding. Abdelgawwad:Bayer AG: Current Employment. Psaroudakis:Bayer AG: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Rivera:Bayer Hispania, S.L.: Current Employment. Brobert:Bayer AB: Ended employment in the past 24 months; Bayer AG: Consultancy. Cohen:Bayer AG: Consultancy, Honoraria, Speakers Bureau; BMS/Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Alexion/Astra Zeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau. Khorana:BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; sanofi: Consultancy, Honoraria; Anthos: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Becattini:Pfizer: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Bayer AG: Consultancy, Honoraria. Lee:Servier: Consultancy; Pfizer: Consultancy, Honoraria; LEO Pharma: Consultancy, Honoraria; Bayer AG: Consultancy, Honoraria; BMS: Honoraria. Carrier:Sanofi: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria; Leo Pharma: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.